Cytokinin signaling
|
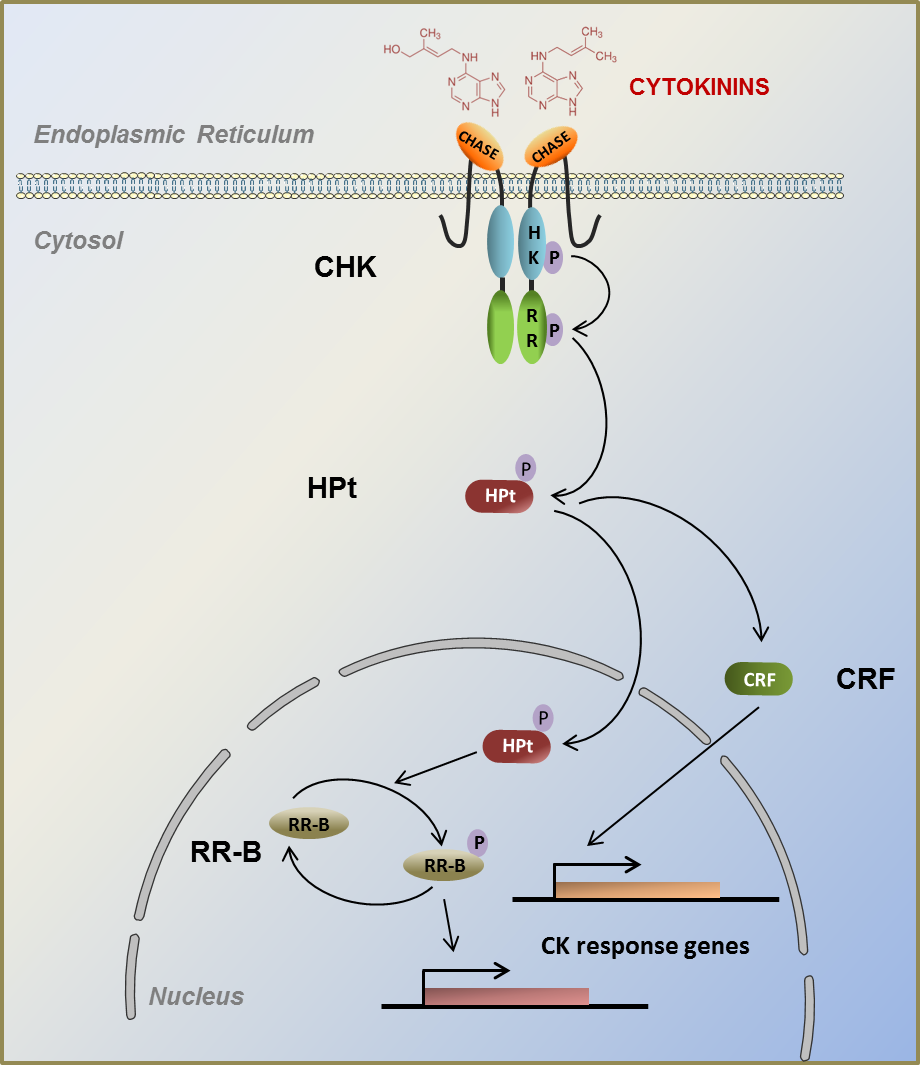
Recently, cytokinin (CK) signaling has emerged as a major factor in plant-microbe and plant-insect interactions. Not only plants synthesize CK, but pathogens such as bacteria or fungi can also produce them. Interestingly, plant- and pathogen-derived CK could inversely affect the plant defense response through the modulation of CK signaling. In plants, CK signaling involves a phosphotransfer cascade composed by histidine-kinase receptors (CHK), histidine phosphotransfer proteins (HPt), response regulators (RR) and CK-response factors (CRF). Research programs performed by the EA2106 BBV team are also focused on the role of CK signaling in apple tree (Malus x domestica) challenged with pathogens or insects. Apple is one of the major fruit crops produced in the world and is submitted to a wide range of pathogens (Erwinia amylovora and Venturia inaequalis, the causal agents of fire blight and apple scab respectively) and pests that caused dramatic damages on cultures. The recent sequenced apple genome allowed us to start identifying the components of CK signaling, especially MdCHK and MdHPt. Now we are currently (i) establishing the functional characterization of the MdCHK in biotic interactions, (ii) determining the MdCHK-MdHPt interaction network. In parallel, we are also studying the “molecular signature” of CK signaling pathways in plant-microbe/insect interactions by bioinformatics tools. |
|  | ↑ Cytokinin signaling pathway is mediated by a two-component system including CHK (cytokinin histidine kinase), HPt (Histidine Phosphotransfer protein), RR (Response Regulator) transcription factors and CRF (cytokinin response factor). |
Contact: Gaëlle Glévarec
Publications:
|
Giron, D. & Glevarec, G. (2014). Cytokinin-induced phenotypes in plant-insect interactions: learning from the bacterial world. J Chem Ecol 40, 826–35. Senoussi, M., Crèche, J., Rideau, M. & Djekoun, A. (2013). Effect of cytokinin in processing at long term on the production of indole alkaloids in periwinkle cellular suspensions. Ann Biol Res 4, 97–101. Héricourt, F., Chefdor, F., Bertheau, L., Tanigawa, M., Maeda, T., Guirimand, G., Courdavault, V., Larcher, M., Depierreux, C., Bénédetti, H., Morabito, D., Brignolas, F., & Carpin, S. (2013). Characterization of histidine-aspartate kinase HK1 and identification of histidine phosphotransfer proteins as potential partners in a Populus multistep phosphorelay. Physiol Plant 149, 188–99. Giron, D., Frago, E., Glevarec, G., Pieterse, C. M. J. & Dicke, M. (2013). Cytokinins as key regulators in plant-microbe-insect interactions: connecting plant growth and defence. Funct Ecol 27, 599–609. Bertheau, L., Miranda, M., Foureau, E., Rojas Hoyos, L. F., Chefdor, F., Héricourt, F., Depierreux, C., Morabito, D., Papon, N. , Clastre, M., Scippa, G. S., Brignolas, F., Courdavault, V. & Carpin, S. (2013). In planta validation of HK1 homodimerization and recruitment of preferential HPt downstream partners involved in poplar multistep phosphorelay systems. Plant Biosyst 147, 991–995. Amini, A., Andreu, F., Glévarec, G., Rideau, M. & Crèche, J. (2012). Down-regulation of the CrHPT1 histidine phosphotransfer protein prevents cytokinin-mediated up-regulation of CrDXR, and CrG10H transcript levels in periwinkle cell cultures. Mol Biol Rep 39, 8491–6. Bertheau, L., Chefdor, F., Guirimand, G., Courdavault, V., Depierreux, C., Morabito, D., Brignolas, F., Héricourt, F. & Carpin, S. (2012). Identification of five B-type response regulators as members of a multistep phosphorelay system interacting with histidine-containing phosphotransfer partners of Populus osmosensor. BMC Plant Biol 12,241. Amini, A., Glévarec, G., Andreu, F., Rideau, M. &Crèche, J. (2009). Low levels of gibberellic acid control the biosynthesis of ajmalicine in Catharanthus roseus cell suspension cultures. Planta Med 75, 187-91. Amini, A., Glévarec, G., Andreu, F., Reverdiau, P., Rideau, M. & Crèche, J. (2008). Effects of phosphatidic acid on cytokinin signal transduction in periwinkle cells. J Plant Growth Regul 27, 394-9. Giron, D., Kaiser, W., Imbault, N. &Casas, J. (2007). Cytokinin-mediated leaf manipulation by a leafminer caterpillar. Biol Lett 3, 340-3. Papon, N., Bremer, J., Vansiri, A., Glévarec, G., Rideau, M. & Crèche, J. (2006). Molecular cloning and expression of a cDNA encoding a hybrid histidine kinase receptor in tropical periwinkle Catharanthus roseus. Plant Biol 8, 731-6. Papon, N., Vansiri, A., Gantet, P., Chénieux, J.-C., Rideau, M. &Crèche, J. (2004). Histidine-containing phosphotransfer domain extinction by RNA interference turns off a cytokinin signalling circuitry in Catharanthus roseus suspension cells. FEBS Lett 558, 85-8. Papon, N., Senoussi, M.M., Andreu, F., Rideau, M., Chénieux, J.-C. &Crèche J. (2004). Cloning of a gene encoding a putative ethylene receptor in Catharanthus roseus and its expression in plant and cell cultures Biol Plant 48, 345-50. Papon, N., Bremer, J., Vansiri, A., Gantet, P. Chénieux, J.-C., Rideau, M. &Crèche, J. (2004). Emergence des voies de transduction de type histidine-aspartate kinase chez les végétaux. Regard sur la biochimie, 2, 23-35. Papon, N., Oudin, A., Vansiri, A., Rideau, M., Chénieux, J.-C. &Crèche, J. (2003). Differential expression of two type-A response regulators in plants and cell cultures of Catharanthus roseus (L.) G. Don. J Exp Bot 54, 1793-5. Papon, N., Clastre, M., Gantet, P., Rideau, M., Chénieux, J.-C. &Crèche, J. (2003). Inhibition of the plant cytokinin transduction pathway by bacterial histidine kinase inhibitors in Catharanthus roseus cell cultures. FEBS Lett 537, 101-5. Papon, N., Clastre, M., Andreu, F., Gantet, P., Rideau, M., & Crèche, J. (2002). Expression analysis in plant and cell suspensions of CrCKR1, a cDNA encoding a histidine kinase receptor homologue in Catharanthus roseus (L.) G. Don. J Exp Bot 53, 1989-90. Papon, N., Oudin, A., Clastre, M., Rideau, M., Chénieux, J.-C. &Crèche, J. (2002). Isolation of CrHPt1, a cDNA encoding a histidine-containing phospho-transfer domain in Catharanthus roseus. Acta Bot Gall 149, 67-77. |
Â
Plant biotechnology
|
Plants are a rich source of natural products that pharmaceutical, cosmetic and food companies explore to develop new drugs, neutraceuticals, perfumes, antioxidants, food additives and various other commodities. Supply of plant materials in nature is regularly problematic and may limit the development of innovative products into commercial success. Many tropical plants are rare in nature, present in limited and inaccessible area, and harvest of essential plant parts (bark, rhizome, roots) threatens conservation of the natural resources. Wild temperate forests are as well a rich source of fine products threatens by climate changes and deforestation. |
 |
 Plant Cell Biotechnology offers alternative system for production of natural products, plant extracts and plant cells. Plant cell suspensions are amenable to large scale culture of dedifferentiated cells accumulating many natural products. Plant tissue culture allows rapid propagation of plant vegetative tissue as a source of healthy stocks for field propagation and for conservation of genetic resources. Hairy root cultures are immortalized roots with continuous growth habits that has the potential to supplies many natural products only found in underground plant parts.
 |
The EA2106 BBV research unit has a long expertise in the development of plant cell biotechnology, in particular from tropical and medicinal plants. Many new cell lines are established from new species for selection of best producing cell lines. Production of natural products is often inhibited by plant growth regulators used for plant cell multiplication (auxin). Optimization of plant growth regulators with respects to metabolic elicitors (sugars, cytokinin, methyljasmonate, ethylene, fungi cell wall fractions) allows stimulation of natural products accumulation. We develop new plant cell platform and improves natural product yields in collaborative research program with industrial partners. The laboratory is fully equipped for plant cell and tissue cultures, with air-filtered facilities for axenic manipulation of plant material and controlled environment growth chambers and shakers. |
|
Selected industrial partnership: “ELIXIR Project” : Production of high-value natural products by plant cell culture technology (2011-2014) Industrial partners : DIANA Group/Symrise (Diana Plant Sciences), Pierre Guérin SA Academic partners : Université de Bourgogne (Laboratoire Génie des Procédés Microbiologiques et Alimentaires), Université de Tours (BBV) Financial support : Oséo/BPI France |
|
Â
Contact: Benoît Saint-Pierre, Joël Crèche
Publications:
|
Etsè, K. D., Aïdam, A. V., Melin, C., Blanc, N., Oudin, A., Courdavault, V., Creche, J. & Lanoue, A. (2014). Optimized genetic transformation of Zanthoxylum zanthoxyloides by Agrobacterium rhizogenes and the production of chelerythrine and skimmiamine in hairy root cultures. Eng Life Sci 14, 95–99. Etsè, K. D., Aïdam, A. V., de Souza, C., Crèche, J. & Lanoue, A. (2011). In vitro propagation of Zanthoxylum zanthoxyloides Lam., an endangered African medicinal plant. Acta Bot Gall 158, 47–55. Senoussi, M.M., Bassa, N. & Crèche, J. (2009). Impact of hypoxia on the growth and alkaloid accumulation in Catharanthus roseus cell suspension. Acta Physiol Plant 31, 359-62. Guillon, S., Trémouillaux-Guiller, J., Pati, P.K. & Gantet, P. (2008). Hairy roots: a powerful tool for plant biotechnological advances. In Bioactive Molecules and Medicinal Plants, pp. 271-283. Edited by K.G. Ramawat & J.-M . Mérillon, Springer. Guillon, S., Gantet, P., Thiersault, M., Rideau, M. & Trémouillaux-Guiller (2008). Hairy Roots of Catharanthus roseus: Efficient Routes to Monomeric Indole Alkaloid Production. In Bioactive Molecules and Medicinal Plants, pp. 285-295. Edited by K.G. Ramawat & J.-M . Mérillon, Springer. Guillon, S., Trémouillaux-Guiller, J., Pati, P.K., Rideau, M. & Gantet, P. (2006). Harnessing the potential of hairy roots: dawn of a new era. Trends Biotechnol 24, 403-9. Guillon, S., Trémouillaux-Guiller, J., Pati, P.K., Rideau, M. & Gantet P. (2006). Hairy root research: recent scenario and exciting prospects. Curr Opin Plant Biol 9, 341-6. Senoussi, M.M., Bassa, N., Crèche, J., Rideau, M. & Chénieux J.-C. (2004). Effects of hypoxia, loganin and BAP on ajmalicine accumulation in cells of Catharanthus roseus (Vinca rosea) In vitro. Damascus University Journal for BASIC SCIENCES 20, 9-17. Ayadi, R. & Trémouillaux-Guiller, J. (2003). Root formation from transgenic calli of Ginkgo biloba. Tree Physiol 23, 713-8. Couillerot, E., Caron, C., Trentesaux, C., Chénieux, J.-C. & Audran, J.-C. (2000). Genetic Transformation of Fagara zanthoxylaides Lam,(Rutaceae). In Biotechnology in Agriculture and Forestry, Vol. 44, pp. 115-124. Edited by Y.P.S. Bajaj. Springer-Verlag Berlin Heildelberg. |
Isoprenoid biosynthesis and regulation
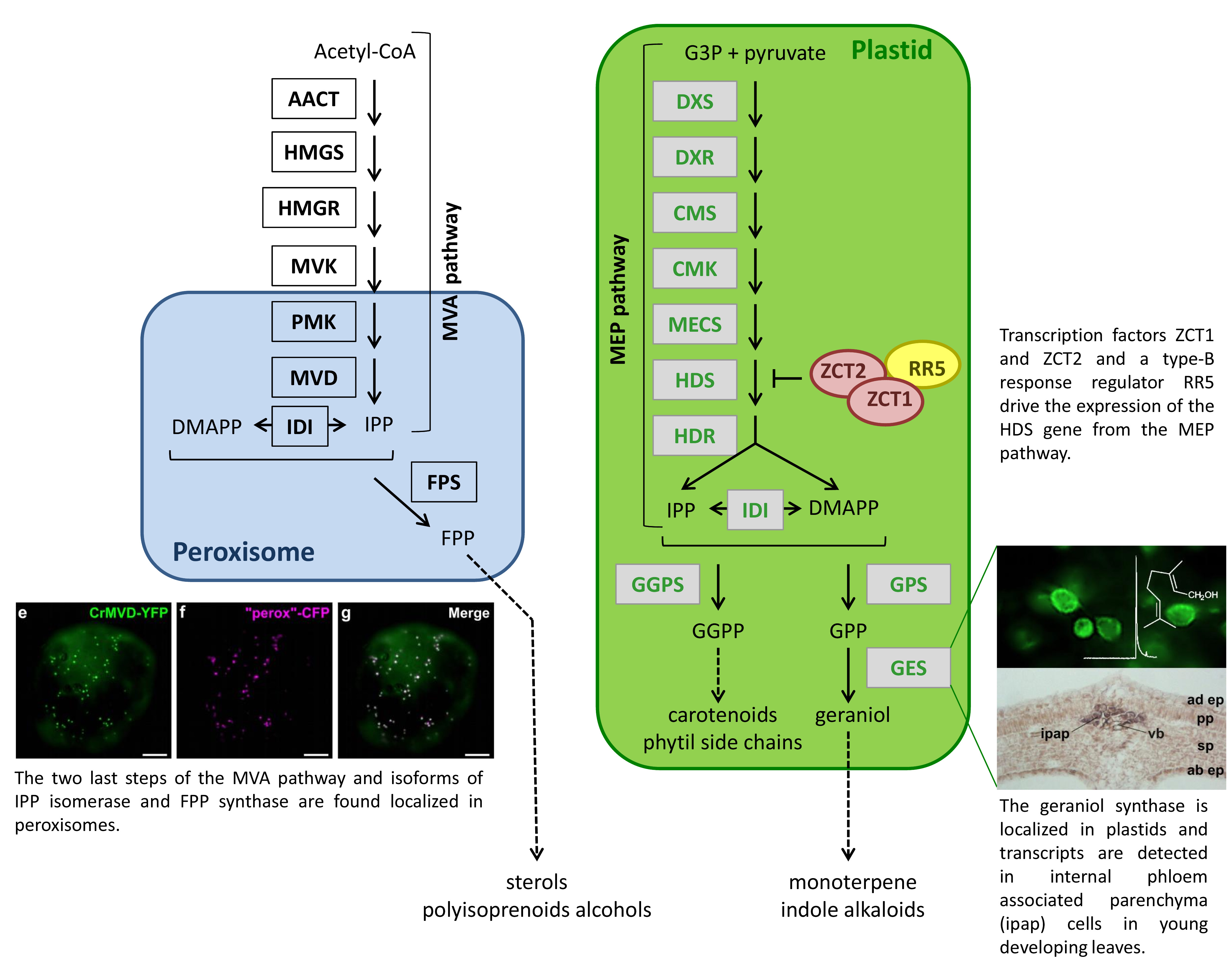
|
Work of EA2106 BBV on isoprenoid metabolism is closely related to the area of focus “Architecture of MIA metabolism”. Isoprenoids (also referred to as terpenoids) are derived from a common C5 precursor, isopentenyl diphosphate (IPP) and its allylic isomer dimethylallyl diphosphate (DMAPP). Two separate IPP forming pathways co-exist in plants: the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway provides the precursors for the synthesis of monoterpenes (and among them the monoterpenes leading to the alkaloids from Catharanthus roseus), carotenoids, apocarotenoids the side chain of chlorophylls, tocopherols and prenylquinones, while the mevalonic acid (MVA) pathway leads to precursors for the formation of triterpenes, sesquiterpenes, phytosterols, ubiquinone, vitamin D and primary metabolites important for cell integrity.  Our research is focused on the elucidation of isoprenoid biosynthesis including the MEP and MVA pathways as well as the next enzymatic steps catalyzed by prenyltransferases and terpene synthases. Moreover, the deciphering of the overall isoprenoid architecture allow us to investigate the regulatory network and to look for transcription factors that would regulate specifically isoprenoid biosynthesis pathway genes and therefore the supply of precursors for alkaloid biosynthesis. During the past years, we established a map at the subcellular and tissular levels of the isoprenoid biosynthesis pathways highlighting the complexity of localization of isoprenoid enzymes. For example, recent subcellular studies have shown that the peroxisome is an additional isoprenoid biosynthetic compartment within plant cells. Our work also led to the characterization of isoprenoid genes and their expression regulation. We therefore isolated few transcription factors that specifically target the MEP pathway genes. |
 Contact: Marc Clastre, Audrey Oudin
Publications:
|
Chebbi, M., Ginis, O., Courdavault, V., GlĂ©varec, G., Lanoue, A., Clastre, M., Papon, N., Gaillard, C., Atanassova, R., St-Pierre, B., Giglioli-Guivarc’h, N., Courtois, M. & Oudin, A. (2014). ZCT1 and ZCT2 transcription factors repress the activity of a gene promoter from the methyl erythritol phosphate pathway in Madagascar periwinkle cells. Journal of Plant Physiology 171, 1510-3. Simkin, A. J., Miettinen, K., Claudel, P., Burlat, V., Guirimand, G., Courdavault, V., Papon, N., Meyer, S., Godet, S., St-Pierre, B., Giglioli-Guivarc'h, N., Fischer, M. J., Memelink, J.  & Clastre, M. (2013). Characterization of the plastidial geraniol synthase from Madagascar periwinkle which initiates the monoterpenoid branch of the alkaloid pathway in internal phloem associated parenchyma. Phytochemistry 85, 36–43. Crouzet, J., Roland, J., Peeters, E., Trombik, T., Ducos, E., Nader, J. & Boutry, M. (2013). NtPDR1, a plasma membrane ABC transporter from Nicotiana tabacum, is involved in diterpene transport. Plant Mol Biol 82, 181–92. Thabet, I., Guirimand, G., Guihur, A., Lanoue, A., Courdavault, V., Papon, N., Bouzid, S., Giglioli-Guivarc’h, N., Simkin, A. J. & Clastre, M. (2012). Characterization and subcellular localization of geranylgeranyl diphosphate synthase from Catharanthus roseus. Mol Biol Rep 39, 3235–43. Guirimand, G., Simkin, A. J., Papon, N., Besseau, S., Burlat, V., St-Pierre, B., Giglioli-Guivarc’h, N., Clastre, M. & Courdavault, V. (2012a). Cycloheximide as a tool to investigate protein import in peroxisomes: a case study of the subcellular localization of isoprenoid biosynthetic enzymes. J Plant Physiol 169, 825–9. Guirimand, G., Guihur, A., Phillips, M. A., Oudin, A., GlĂ©varec, G., Melin, C., Papon, N., Clastre, M., St-Pierre, B., RodrĂguez-ConcepciĂłn, M., Burlat, V. & Courdavault, V. (2012b). A single gene encodes isopentenyl diphosphate isomerase isoforms targeted to plastids, mitochondria and peroxisomes in Catharanthus roseus. Plant Mol Biol 79, 443–59. Ginis, O., Courdavault, V., Melin, C., Lanoue, A., Giglioli-Guivarc’h, N., St-Pierre, B., Courtois, M. & Oudin, A. (2012a). Molecular cloning and functional characterization of Catharanthus roseus hydroxymethylbutenyl 4-diphosphate synthase gene promoter from the methyl erythritol phosphate pathway. Mol Biol Rep 39, 5433–47. Ginis, O., Oudin, A., Guirimand, G., Chebbi, M., Courdavault, V., GlĂ©varec, G., Papon, N., Crèche, J. & Courtois, M. (2012b). A type-B response regulator drives the expression of the hydroxymethylbutenyl diphosphate synthase gene in periwinkle. J Plant Physiol 169, 1571-4. Guirimand, G., Guihur, A., Phillips, M.A., Oudin, A., GlĂ©varec, G., Mahroug, S., Melin, C., Papon, N., Clastre, M., Giglioli-Guivarc'h, N., St-Pierre, B., RodrĂguez-ConcepciĂłn, M., Burlat, V. & Courdavault, V.(2012). Triple subcellular targeting of isopentenyl diphosphate isomerases encoded by a single gene. Plant Signaling & Behavior 7,1495-7. Thabet, I., Guirimand, G., Courdavault, V., Papon, N., Godet, S., Dutilleul, C., Bouzid, S., Giglioli-Guivarc’h, N., Clastre, M. & Simkin, A. J. (2011). The subcellular localization of periwinkle farnesyl diphosphate synthase provides insight into the role of peroxisome in isoprenoid biosynthesis. J Plant Physiol 168, 2110–6. Simkin, A. J., Guirimand, G., Papon, N., Courdavault, V., Thabet, I., Ginis, O., Bouzid, S., Giglioli-Guivarc’h, N. & Clastre, M. (2011). Peroxisomal localisation of the final steps of the mevalonic acid pathway in planta. Planta 234, 903–14. Clastre, M., Papon, N., Courdavault, V., Giglioli-Guivarc’h, N., St-Pierre, B. & Simkin, A. J. (2011). Subcellular evidence for the involvement of peroxisomes in plant isoprenoid biosynthesis. Plant Signal Behav 6, 2044–6. Guirimand, G., Burlat, V., Oudin, A., Lanoue, A., St-Pierre, B. & Courdavault, V.  (2009). Optimization of the transient transformation of Catharanthus roseus cells by particle bombardment and its application to the subcellular localization of hydroxymethylbutenyl 4-diphosphate synthase and geraniol 10-hydroxylase. Plant Cell Rep 28, 1215-34. Oudin, A., Mahroug, S., Courdavault, V., Hervouet, N., Zelwer, C., RodrĂguez-ConcepciĂłn, M., St-Pierre, B. & Burlat V. (2007). Spatial distribution and hormonal regulation of gene products from methyl erythritol phosphate and monoterpene-secoiridoid pathways inCatharanthus roseus. Plant Mol Biol 65, 13-30. Oudin. A., Courtois. M., Rideau. M. & Clastre. M. (2007). The iridoid pathway in Catharanthus roseus alkaloid biosynthesis. Phytochem Rev 6, 259-76. Papon, N., Bremer, J., Vansiri, A., Andreu, F., Rideau, M. & Crèche, J. (2005). Cytokinin and ethylene control indole alkaloid production at the level of the MEP/terpenoid pathway in Catharanthus roseus suspension cells. Planta Med 71, 572-4. Mincheva, Z., Courtois, M., Andreu, F., Rideau, M. & Viaud-Massuard, M.-C. (2005). Fosmidomycin analogues as inhibitors of monoterpenoid indole alkaloid production in Catharanthus roseus cells. Phytochemistry 66, 1797-803. Courtois, M., Mincheva, Z., Andreu, F., Rideau, M. & Viaud-Massuard, M.-C. (2004). Synthesis and biological evaluation with plant cells of new fosmidomycin analogues containing a benzoxazolone or oxazolopyridinone ring. J Enzyme Inhib Med Chem 19, 559-65. Mincheva, Z., Courtois, M., Crèche, J., Rideau, M. & Viaud-Massuard M.-C. (2004). One-pot synthesis of functionalized 4,5-dihydroisoxazole derivatives via nitrile oxides and biological evaluation with plant cells. Bioorg Med Chem 12, 191-7. Burlat, V., Oudin, A., Courtois, M., Rideau, M., & St-Pierre, B. (2004). Co-expression of three MEP pathway genes and geraniol 10-hydroxylase in internal phloem parenchyma of Catharanthus roseus implicates multicellular translocation of intermediates during the biosynthesis of monoterpene indole alkaloids and isoprenoid-derived primary metabolites. Plant J 38, 131-41. Oudin, A., Veau, B., Courtois, M., Guivarc’h, N., Chahed, K., ChĂ©nieux, J.-C.,  Rideau, M. & Clastre, M. (2001). Architecture des voies terpĂ©niques impliquĂ©es dans la biosynthèse des alcaloĂŻdes chez la pervenche de Madagascar. Dans: Des modèles biologiques Ă l’amĂ©lioration des plantes, S. Hamon ed, p 445-464 (ISBN : 2-7099-1472-7). Veau, B., Courtois, M., Oudin, A., ChĂ©nieux, J.-C., Rideau, M. & Clastre, M. (2000). Cloning and expression of cDNAs encoding two enzymes of the MEP pathway in Catharanthus roseus. Biochim Biophys Acta 1517, 159-63. Chahed, K., Oudin, A., Guivarc'h, N., Hamdi, S., ChĂ©nieux, J.-C., Rideau, M. & Clastre, M. (2000). 1-deoxyxylulose-D-xylulose 5-phosphate synthase from periwinkle : cDNA identification and induced gene expression in terpenoid indole alkaloid-producing cells. Plant Physiol Biochem 38, 559-66. |