Architecture of MIA metabolism

| Monoterpene Indole alakoids originate from complex biosynthetic pathways exhibiting both thighly regulation processes and high degrees of organisation in planta. Over the last years, the EA2106 research team has engaged (1) program of MIA biosynthetic gene identification as well as (2) the in depth characterization of MIA and terpene biosynthetic pathway compartmentalization at the cellular and subcellular levels focusing on the medicinal plant Catharanthus roseus |
| Triple subcellular targeting of isopentenyl diphosphate isomerase in Catharanthus roseus. Plant Mol. Biol. (2012): 79:443-459 → |
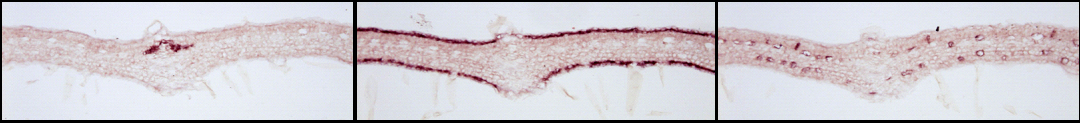
| For instance, the combined analysis of the cellular distribution of gene transcripts by RNA in situ hybridization and proteins by immunological approaches leds to the identification of al least four distinct cells types housing the C. roseus MIA biosynthetic pathway in folio: (1) cells of the internal phloem associated parenchyma for the early steps of the pathway and notably the methylerythritol pathway, (2) cells of both adaxial and abaxial leaf epidermis for the central steps, (3) the specialized cells idioblasts and laticifers for the final steps of MIA biosynthesis. Such original comparmentalization raises therefore questions about the involvement of metabolite transport in the whole MIA biosynthesis regulation. |
| (1) Early steps | (2) Intermediate Steps | (3) Final Steps |
 |
||
Â
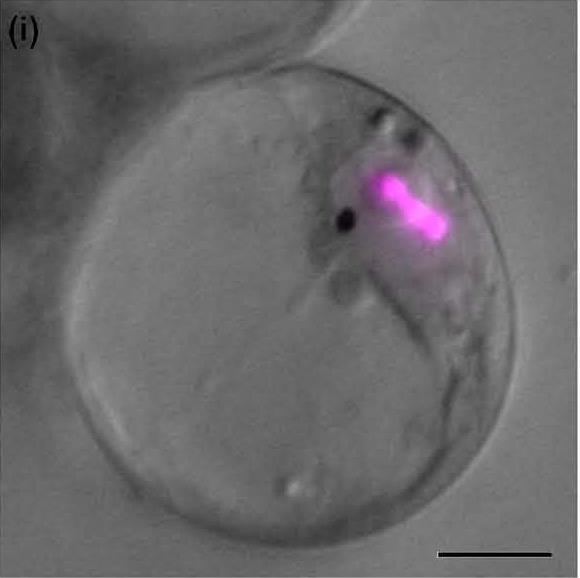
An integrated model of the MIA biosynthetic pathway organisation is currently developed by studying the enzyme subcellular localization using GFP imaging and immunological approaches. This notably points out the physical sequestration of the strictosine synthase (STR) in the vacuole and of the stricosidine glucosidase (SGD) in the nucleus engendering a plant defensive mechanism called “nuclear time bomb” as illustrated below. Such studies also enable us to establish that peroxisomes play fondamental roles in terpenoid biosynthesis by housing the last enzymes of the mevalonate pathway.Â
|  Subcellular organisation of MIA biosynthetic enzmes in epidermis of C. roseus leaves. The aglycon realesed following stricosidine degluscosylation is a highly reactive compounds causing massive protein reticulation during pathogen attacks.→ |  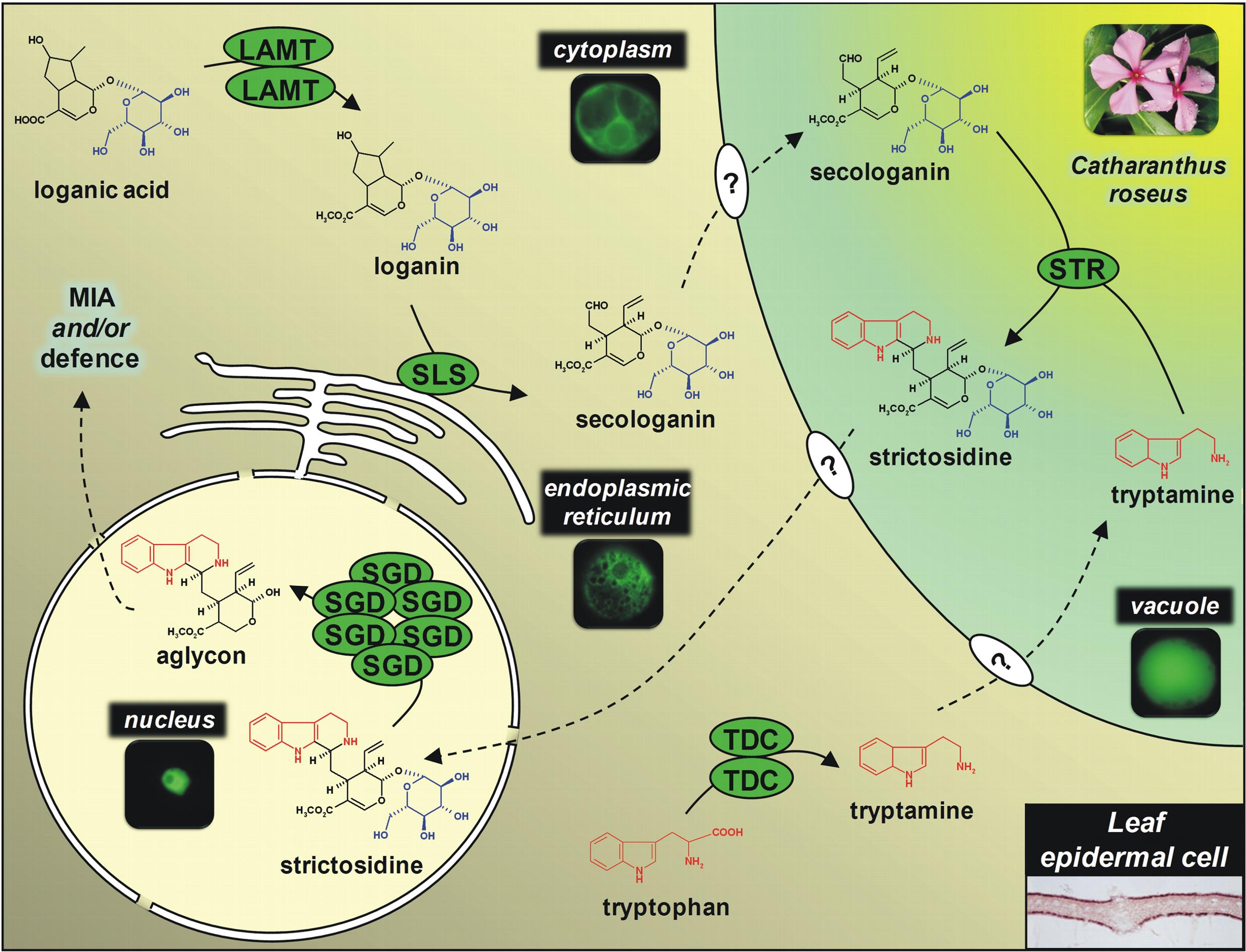 |
| SGD resides in the nucleus as multimers  ↓ | |
  |
Contact: Vincent Courdavault
Publications:
|
DugĂ© de Bernonville, T., Clastre, M., Besseau, S., Oudin, A., Burlat, V., GlĂ©varec, G., Lanoue, A., Papon, N., Giglioli-Guivarc’h, N., St-Pierre, B. & Courdavault, V. (2014b). Phytochemical genomics of the Madagascar periwinkle: Unravelling the last twists of the alkaloid engine. Phytochemistry In press. Courdavault, V., Papon, N., Clastre, M., Giglioli-Guivarc’h, N., St-Pierre, B., Burlat, V. (2014). A look inside an alkaloid multisite plant: the Catharanthus logistics. Current opinion in plant Biology 19, 43-50. St-Pierre, B., Besseau, S., Clastre, M., Courdavault, V., Courtois, M., Crèche, J., Ducos, E., de Bernonville, T. D., Dutilleul, C., Glevarec, G., Imbault, N., Lanoue, A., Oudin, A., Papon, N., Pichon, O. & Giglioli-Guivarc’h, N. (2013). New Light on Alkaloid Biosynthesis and Future Prospects. Adv Bot Res 68, 73-109. Besseau, S., Kellner, F., Lanoue, A., Thamm, A. M. K., Salim, V., Schneider, B., Geu-Flores, F., Höfer, R., Guirimand, G., Guihur, A., Oudin, A., Glevarec, G., Foureau, E., Papon, N., Clastre, M., Giglioli-Guivarc'h, N., St-Pierre, B., Werck-Reichhart, D., Burlat, V., De Luca, V., O'Connor, S. E. & Courdavault, V. (2013). A pair of tabersonine 16-hydroxylases initiates the synthesis of vindoline in an organ-dependent manner in Catharanthus roseus. Plant Physiol 163, 1792–803. Simkin, A. J., Miettinen, K., Claudel, P., Burlat, V., Guirimand, G., Courdavault, V., Papon, N., Meyer, S., Godet, S., St-Pierre, B., Giglioli-Guivarc'h, N., Fischer, M. J., Memelink, J.  & Clastre, M. (2013). Characterization of the plastidial geraniol synthase from Madagascar periwinkle which initiates the monoterpenoid branch of the alkaloid pathway in internal phloem associated parenchyma. Phytochemistry 85, 36–43. Geu-Flores, F., Sherden, N. H., Courdavault, V., Burlat, V., Glenn, W. S., Wu, C., Nims, E., Cui, Y. & O’Connor, S. E. (2012). An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis. Nature 492, 138–42. Guirimand, G., Simkin, A. J., Papon, N., Besseau, S., Burlat, V., St-Pierre, B., Giglioli-Guivarc’h, N., Clastre, M. & Courdavault, V. (2012a). Cycloheximide as a tool to investigate protein import in peroxisomes: a case study of the subcellular localization of isoprenoid biosynthetic enzymes. J Plant Physiol 169, 825–9. Guirimand, G., Guihur, A., Phillips, M. A., Oudin, A., GlĂ©varec, G., Melin, C., Papon, N., Clastre, M., St-Pierre, B., RodrĂguez-ConcepciĂłn, M., Burlat, V. & Courdavault, V. (2012b). A single gene encodes isopentenyl diphosphate isomerase isoforms targeted to plastids, mitochondria and peroxisomes in Catharanthus roseus. Plant Mol Biol 79, 443–59. Guirimand, G., Guihur, A., Phillips, M.A., Oudin, A., GlĂ©varec, G., Mahroug, S., Melin, C., Papon, N., Clastre, M., Giglioli-Guivarc'h, N., St-Pierre, B., RodrĂguez-ConcepciĂłn, M., Burlat, V. & Courdavault, V. (2012c). Triple subcellular targeting of isopentenyl diphosphate isomerases encoded by a single gene. Plant Signaling & Behavior 7,1495-7. Makhzoum, A., Petit-Paly, G., St Pierre, B. & Bernards, M. A. (2011). Functional analysis of the DAT gene promoter using transient Catharanthus roseus and stable Nicotiana tabacum transformation systems. Plant Cell Rep 30, 1173–82. Guirimand, G., Guihur, A., Ginis, O., Poutrain, P., HĂ©ricourt, F., Oudin, A., Lanoue, A., St-Pierre, B., Burlat, V. & Courdavault, V. (2011a). The subcellular organization of strictosidine biosynthesis in Catharanthus roseus epidermis highlights several trans-tonoplast translocations of intermediate metabolites. FEBS J 278, 749–63. Guirimand, G., Guihur, G., Poutrain, P., HĂ©ricourt, F., Mahroug, S., St-Pierre, B., Burlat V. &  Courdavault, V. (2011b). Spatial organization of the vindoline biosynthetic pathway in Catharanthus roseus. Journal of Plant Physiology 168, 549-57. Poutrain, P., Guirimand, G., Mahroug, S., Burlat, V., Melin, C., Ginis, O., Oudin, A., Giglioli-Guivarc’h, N., Pichon, O. & Courdavault, V. (2011). Molecular cloning and characterisation of two calmodulin isoforms of the Madagascar periwinkle Catharanthus roseus. Plant Biol (Stuttg) 13, 36–41. Guirimand, G., Courdavault, V., Lanoue, A., Mahroug, S., Guihur, A., Blanc, N., Giglioli-Guivarc’h, N., St-Pierre, B. & Burlat, V. (2010). Strictosidine activation in Apocynaceae: towards a “nuclear time bomb”? BMC Plant Biol 10, 182. Guirimand, G., Burlat, V., Oudin, A., Lanoue, A., St-Pierre, B. & Courdavault, V. (2009). Optimization of the transient transformation of Catharanthus roseus cells by particle bombardment and its application to the subcellular localization of hydroxymethylbutenyl 4-diphosphate synthase and geraniol 10-hydroxylase. Plant Cell Reports 28, 1215-34. |
Â