Architecture of MIA metabolism

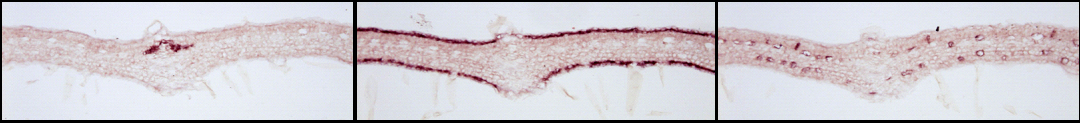
| Monoterpene Indole alakoids originate from complex biosynthetic pathways exhibiting both thighly regulation processes and high degrees of organisation in planta. Over the last years, the EA2106 research team has engaged (1) program of MIA biosynthetic gene identification as well as (2) the in depth characterization of MIA and terpene biosynthetic pathway compartmentalization at the cellular and subcellular levels focusing on the medicinal plant Catharanthus roseus |
| Triple subcellular targeting of isopentenyl diphosphate isomerase in Catharanthus roseus. Plant Mol. Biol. (2012): 79:443-459 â |
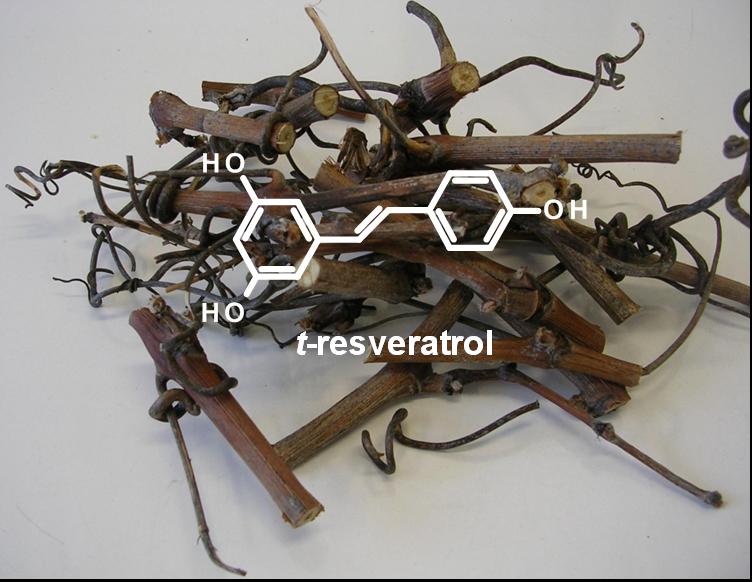
| For instance, the combined analysis of the cellular distribution of gene transcripts by RNA in situ hybridization and proteins by immunological approaches leds to the identification of al least four distinct cells types housing the C. roseus MIA biosynthetic pathway in folio: (1) cells of the internal phloem associated parenchyma for the early steps of the pathway and notably the methylerythritol pathway, (2) cells of both adaxial and abaxial leaf epidermis for the central steps, (3) the specialized cells idioblasts and laticifers for the final steps of MIA biosynthesis. Such original comparmentalization raises therefore questions about the involvement of metabolite transport in the whole MIA biosynthesis regulation. |
| (1) Early steps | (2) Intermediate Steps | (3) Final Steps |
 |
||
Â
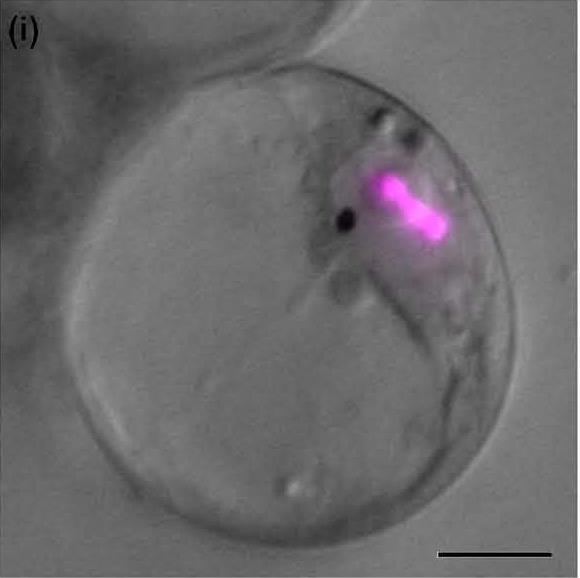
An integrated model of the MIA biosynthetic pathway organisation is currently developed by studying the enzyme subcellular localization using GFP imaging and immunological approaches. This notably points out the physical sequestration of the strictosine synthase (STR) in the vacuole and of the stricosidine glucosidase (SGD) in the nucleus engendering a plant defensive mechanism called ânuclear time bombâ as illustrated below. Such studies also enable us to establish that peroxisomes play fondamental roles in terpenoid biosynthesis by housing the last enzymes of the mevalonate pathway.Â
| Â Subcellular organisation of MIA biosynthetic enzmes in epidermis of C. roseus leaves. The aglycon realesed following stricosidine degluscosylation is a highly reactive compounds causing massive protein reticulation during pathogen attacks.â | Â  |
| SGD resides in the nucleus as multimers  â | |
  |
Contact: Vincent Courdavault
Publications:
|
DugĂ© de Bernonville, T., Clastre, M., Besseau, S., Oudin, A., Burlat, V., GlĂ©varec, G., Lanoue, A., Papon, N., Giglioli-Guivarcâh, N., St-Pierre, B. & Courdavault, V. (2014b). Phytochemical genomics of the Madagascar periwinkle: Unravelling the last twists of the alkaloid engine. Phytochemistry In press. Courdavault, V., Papon, N., Clastre, M., Giglioli-Guivarcâh, N., St-Pierre, B., Burlat, V. (2014). A look inside an alkaloid multisite plant: the Catharanthus logistics. Current opinion in plant Biology 19, 43-50. St-Pierre, B., Besseau, S., Clastre, M., Courdavault, V., Courtois, M., CrĂšche, J., Ducos, E., de Bernonville, T. D., Dutilleul, C., Glevarec, G., Imbault, N., Lanoue, A., Oudin, A., Papon, N., Pichon, O. & Giglioli-Guivarcâh, N. (2013). New Light on Alkaloid Biosynthesis and Future Prospects. Adv Bot Res 68, 73-109. Besseau, S., Kellner, F., Lanoue, A., Thamm, A. M. K., Salim, V., Schneider, B., Geu-Flores, F., Höfer, R., Guirimand, G., Guihur, A., Oudin, A., Glevarec, G., Foureau, E., Papon, N., Clastre, M., Giglioli-Guivarc'h, N., St-Pierre, B., Werck-Reichhart, D., Burlat, V., De Luca, V., O'Connor, S. E. & Courdavault, V. (2013). A pair of tabersonine 16-hydroxylases initiates the synthesis of vindoline in an organ-dependent manner in Catharanthus roseus. Plant Physiol 163, 1792â803. Simkin, A. J., Miettinen, K., Claudel, P., Burlat, V., Guirimand, G., Courdavault, V., Papon, N., Meyer, S., Godet, S., St-Pierre, B., Giglioli-Guivarc'h, N., Fischer, M. J., Memelink, J.  & Clastre, M. (2013). Characterization of the plastidial geraniol synthase from Madagascar periwinkle which initiates the monoterpenoid branch of the alkaloid pathway in internal phloem associated parenchyma. Phytochemistry 85, 36â43. Geu-Flores, F., Sherden, N. H., Courdavault, V., Burlat, V., Glenn, W. S., Wu, C., Nims, E., Cui, Y. & OâConnor, S. E. (2012). An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis. Nature 492, 138â42. Guirimand, G., Simkin, A. J., Papon, N., Besseau, S., Burlat, V., St-Pierre, B., Giglioli-Guivarcâh, N., Clastre, M. & Courdavault, V. (2012a). Cycloheximide as a tool to investigate protein import in peroxisomes: a case study of the subcellular localization of isoprenoid biosynthetic enzymes. J Plant Physiol 169, 825â9. Guirimand, G., Guihur, A., Phillips, M. A., Oudin, A., GlĂ©varec, G., Melin, C., Papon, N., Clastre, M., St-Pierre, B., RodrĂguez-ConcepciĂłn, M., Burlat, V. & Courdavault, V. (2012b). A single gene encodes isopentenyl diphosphate isomerase isoforms targeted to plastids, mitochondria and peroxisomes in Catharanthus roseus. Plant Mol Biol 79, 443â59. Guirimand, G., Guihur, A., Phillips, M.A., Oudin, A., GlĂ©varec, G., Mahroug, S., Melin, C., Papon, N., Clastre, M., Giglioli-Guivarc'h, N., St-Pierre, B., RodrĂguez-ConcepciĂłn, M., Burlat, V. & Courdavault, V. (2012c). Triple subcellular targeting of isopentenyl diphosphate isomerases encoded by a single gene. Plant Signaling & Behavior 7,1495-7. Makhzoum, A., Petit-Paly, G., St Pierre, B. & Bernards, M. A. (2011). Functional analysis of the DAT gene promoter using transient Catharanthus roseus and stable Nicotiana tabacum transformation systems. Plant Cell Rep 30, 1173â82. Guirimand, G., Guihur, A., Ginis, O., Poutrain, P., HĂ©ricourt, F., Oudin, A., Lanoue, A., St-Pierre, B., Burlat, V. & Courdavault, V. (2011a). The subcellular organization of strictosidine biosynthesis in Catharanthus roseus epidermis highlights several trans-tonoplast translocations of intermediate metabolites. FEBS J 278, 749â63. Guirimand, G., Guihur, G., Poutrain, P., HĂ©ricourt, F., Mahroug, S., St-Pierre, B., Burlat V. &  Courdavault, V. (2011b). Spatial organization of the vindoline biosynthetic pathway in Catharanthus roseus. Journal of Plant Physiology 168, 549-57. Poutrain, P., Guirimand, G., Mahroug, S., Burlat, V., Melin, C., Ginis, O., Oudin, A., Giglioli-Guivarcâh, N., Pichon, O. & Courdavault, V. (2011). Molecular cloning and characterisation of two calmodulin isoforms of the Madagascar periwinkle Catharanthus roseus. Plant Biol (Stuttg) 13, 36â41. Guirimand, G., Courdavault, V., Lanoue, A., Mahroug, S., Guihur, A., Blanc, N., Giglioli-Guivarcâh, N., St-Pierre, B. & Burlat, V. (2010). Strictosidine activation in Apocynaceae: towards a ânuclear time bombâ? BMC Plant Biol 10, 182. Guirimand, G., Burlat, V., Oudin, A., Lanoue, A., St-Pierre, B. & Courdavault, V. (2009). Optimization of the transient transformation of Catharanthus roseus cells by particle bombardment and its application to the subcellular localization of hydroxymethylbutenyl 4-diphosphate synthase and geraniol 10-hydroxylase. Plant Cell Reports 28, 1215-34. |
Â
Polyphenol metabolism
|
Phenylpropanoids are ubiquitous phenylalanine-derived plant natural products involved in various physiological process at the interface of plant/environment interaction. They form a huge class of phytochemicals that comprise, among others, polyphenols such as flavonoids or stilbenes. Part of the EA2106 research team program focuses on the phenylpropanoid metabolism of apple tree and grape, two species of agronomic interest in the Loire valley. Using integrative approaches (molecular biology, phytochemistry, bioassays and field experiments) we aim to elucidate the phenylpropanoids biosynthesis pathways and its function in plant interactions with pathogens and pests. |
 |
Elucidation of dihydrochalcone metabolism in apple tree Dihydrochalcones represent the major flavonoid subgroup in apple green tissues and are considered as protective compounds. A global functional genetic approach is used to both identify genes implicated in the biosynthesis pathway, and disturb dihydrochalcones accumulation in planta to access its functions. Expression of candidate genes are transiently modulate using virus induced gene silencing (VIGS) or viral overexpression systems. â Apple tree leaves yellowing after down regulation of RuBisCo expression by VIGS |
|
Stilbene as bioactive compounds in grape ACTISARM (PI: Arnaud Lanoue, Project RĂ©gion Centre) aims to develop stilbene extracts from grape canes as alternative fungicides. In collaboration with IFV (French Vine and Wine Institute), vineyard experiments are conducted to test the potential of stilbene extracts to fight downy mildew. In parallel we reported the activity of resveratrol derivatives against common agents of human mycoses such as Candida yeasts (HouillĂ© et al., 2014). Recently, VITITERROIR (PI: Samuel Leturcq, CNRS, UMR 6173 CITERES Project RĂ©gion Centre) was launched to study the influence of the âterroirâ on polyphenol content in grape. Grappe canes as a source of resveratrol â |
|
Contact: Arnaud Lanoue, SĂ©bastien Besseau
Publications:
|
HouillĂ©, B., Besseau, S., Courdavault, V., Oudin, A., GlĂ©varec, G., Delanoue, G., GuĂ©rin, L., Simkin, A.J., Papon, N., Clastre, M., Giglioli-Guivarc'h, N. & Lanoue, A. (2015). Biosynthetic Origin of E-Resveratrol Accumulation in Grape Canes during Postharvest Storage. J Agric Food Chem 63, 1631â38. HouillĂ©, B., Papon, N., Boudesocque, L., Bourdeaud, E., Besseau, S., Courdavault, V., Enguehard-Gueiffier, C., Delanoue, G., GuĂ©rin, L., Bouchara, J.-P., Clastre, M., Giglioli-Guivarcâh, N., Guillard, J. & Lanoue, A. (2014). Antifungal Activity of Resveratrol Derivatives against Candida Species. J Nat Prod 77, 1658â62. Gaucher, M., DugĂ© de Bernonville, T., Lohou, D., Guyot, S., Guillemette, T., Brisset, M.-N. & Dat, J. F. (2013a). Histolocalization and physico-chemical characterization of dihydrochalcones: Insight into the role of apple major flavonoids. Phytochemistry 90, 78â89. Gaucher, M., DugĂ© de Bernonville, T., Guyot, S., Dat, J. F. & Brisset, M.-N. (2013b). Same ammo, different weapons: enzymatic extracts from two apple genotypes with contrasted susceptibilities to fire blight (Erwinia amylovora) differentially convert phloridzin and phloretin in vitro. Plant Physiol Biochem 72, 178â89. Grienenberger, E., Besseau, S., Geoffroy, P., Debayle, D., Heintz, D., Lapierre, C., Pollet, B., Heitz, T., Legrand, M. (2009). A BAHD acyltransferase is expressed in the tapetum of Arabidopsis anthers and is involved in the synthesis of hydroxycinnamoyl spermidines. Plant J 58, 246-59. |
Yeast Engineering
  |
Yeast metabolic engineering is the enabling science of development of efficient cell factories for the production of fuels, chemicals, pharmaceuticals, and food ingredients through fungal fermentations. The yeast Saccharomyces cerevisiae is already used for the production of a wide range of valuable compounds. In addition, various Candida species (e.g. C. tropicalis, C. maltosa, C. famata, C. rugosa, C. guilliermondii) represent powerful biotechnological models for bioconversions or industrial production of value-added metabolites. The efficient ability of certain Candida strains to metabolize plant by-products is now being evaluated by a number of research teams in order to produce biocompounds such as antibiotics, vitamins, complex alkanes and biofuel. The trend towards this Candida âwhite biotechnologyâ originates from the extraordinary capacities of a small number of Candida species to metabolize C5 sugars from hemicellulosic waste. Part of the EA2106 BBV research programs is now dedicated to the development of genetic tools and strategies aiming at optimizing the production of (i) pharmaceutical compounds by transferring branches of plant biosynthetic pathways in S. cerevisiae and (ii) sweeteners and biofuel by Candida sp. engineered strains.  |
| Application of CFP, GFP, YFP and mCherry as a series of four fluorescent visual selection markers in Candida sp. See Papon et al. (2012) Microbiology. 158: 585-600. |
Â
 |
Engineering Candida yeasts for ethanol production.(A) Production of ethanol from plant hemicellulose hydrolysates of plant biomass. (B) Combination of various strategies to rationally engineer Candida cells for high production of ethanol from xylose. See Papon et al. (2014) Trends in Biotechnology. 32: 167-168. |
Contact: Nicolas Papon
Publications:
|
Defosse, T. A., Sharma, A., Mondal, A.K., DugĂ© de Bernonville, T., LatgĂ©, J.-P., Calderone, R., Giglioli-Guivarc'h, N., Courdavault, V., Clastre, M. & Papon, N. (2015). Hybrid histidine kinases in pathogenic fungi. Mol Microbiol doi:10.1111/mmi.12911 Defosse, T. A., Melin, C., Obando Montoya, E. J., Lanoue, A., Foureau, E., GlĂ©varec, G., Oudin, A., Simkin, A. J., CrĂšche, J., AtehortĂča, L., Giglioli-Guivarcâh, N., Clastre, M., Courdavault, V. & Papon, N. (2014). A new series of vectors for constitutive, inducible or repressible gene expression in Candida guilliermondii. J Biotechnol 180, 37â42. HouillĂ©, B., Papon, N., Boudesocque, L., Bourdeaud, E., Besseau, S., Courdavault, V., Enguehard-Gueiffier, C., Delanoue, G., GuĂ©rin, L., Bouchara, J.-P., Clastre, M., Giglioli-Guivarcâh, N., Guillard, J. & Lanoue, A. (2014). Antifungal Activity of Resveratrol Derivatives against Candida Species. J Nat Prod 77, 1658â62. Papon, N., Courdavault, V., Lanoue, A., Clastre, M. & Brock, M.(2014a). Illuminating fungal infections with bioluminescence. PLoS Pathog 10, e1004179. Papon, N., Courdavault, V., & Clastre, M. (2014b). Biotechnological potential of the fungal CTG clade species in the synthetic biology era. Trends in Biotechnology 32, 167-8. Obando Montoya, E. J., MĂ©lin, C., Blanc, N., Lanoue, A., Foureau, E., Boudesocque, L., Prie, G., Simkin, A. J., CrĂšche, J., AtehortĂča, L., Giglioli-Guivarcâh, N., Clastre, M., Courdavault, V. & Papon, N. (2014). Disrupting the methionine biosynthetic pathway in Candida guilliermondii: characterization of the MET2 gene as counter-selectable marker. Yeast 31, 243â51. Foureau, E., Clastre, M., Montoya, E. J. O., Besseau, S., Oudin, A., GlĂ©varec, G., Simkin, A. J., CrĂšche, J., AtehortĂča, L., Giglioli-Guivarcâh, N., Courdavault, V. & Papon, N. (2014). Subcellular localization of the histidine kinase receptors Sln1p, Nik1p and Chk1p in the yeast CTG clade species Candida guilliermondii. Fungal Genet Biol 65, 25â36. Papon, N., Courdavault, V., Clastre, M. & Bennett, R. J. (2013b). Emerging and emerged pathogenic Candida species: beyond the Candida albicans paradigm. PLoS Pathog 9, e1003550. Papon, N., Savini, V., Lanoue, A., Simkin, A. J., CrĂšche, J., Giglioli-Guivarcâh, N., Clastre, M., Courdavault, V. & Sibirny, A. A. (2013a). Candida guilliermondii: biotechnological applications, perspectives for biological control, emerging clinical importance and recent advances in genetics. Curr Genet 59, 73â90. Foureau, E., Courdavault, V., Rojas, L. F., Dutilleul, C., Simkin, A. J., CrĂšche, J., AtehortĂča, L., Giglioli-Guivarcâh, N., Clastre, M. & Papon, N. (2013a). Efficient gene targeting in a Candida guilliermondii non-homologous end-joining pathway-deficient strain. Biotechnol Lett 35, 1035â43. Foureau, E., Courdavault, V., Simkin, A. J., Sibirny, A. A., CrĂšche, J., Giglioli-Guivarcâh, N., Clastre, M. & Papon, N. (2013b). Transformation of Candida guilliermondii wild-type strains using the Staphylococcus aureus MRSA 252 ble gene as a phleomycin-resistant marker. FEMS Yeast Res 13, 354â8. Foureau, E., Courdavault, V., Navarro GallĂłn, S. M., Besseau, S., Simkin, A. J., CrĂšche, J., AtehortĂča, L., Giglioli-Guivarcâh, N., Clastre, M. & Papon, N. (2013c). Characterization of an autonomously replicating sequence in Candida guilliermondii. Microbiol Res 168, 580â8. Foureau, E., Clastre, M., Millerioux, Y., Simkin, A. J., Cornet, L., Dutilleul, C., Besseau, S., Marais, E., Melin, C. , Guillard, J., CrĂšche, J., Giglioli-Guivarc'h, N., Courdavault, V. & Papon, N. (2012). A TRP5/5-fluoroanthranilic acid counter-selection system for gene disruption in Candida guilliermondii. Curr Genet 58, 245â54. Papon, N., Courdavault, V., Clastre, M., Simkin, A. J., CrĂšche, J. & Giglioli-Guivarcâh, N. (2012). Deus ex Candida genetics: overcoming the hurdles for the development of a molecular toolbox in the CTG clade. Microbiology 158, 585â600. Millerioux, Y., Clastre, M., Simkin, A. J., Courdavault, V., Marais, E., Sibirny, A. A., NoĂ«l, T., CrĂšche, J., Giglioli-Guivarcâh, N. & Papon, N. (2011a). Drug-resistant cassettes for the efficient transformation of Candida guilliermondii wild-type strains. FEMS Yeast Res 11, 457â63. Millerioux, Y., Clastre, M., Simkin, A. J., Marais, E., Sibirny, A. A., NoĂ«l, T., CrĂšche, J., Giglioli-Guivarcâh, N. & Papon, N. (2011b). Development of a URA5 integrative cassette for gene disruption in the Candida guilliermondii ATCC 6260 strain. J Microbiol Methods 84, 355â8. Courdavault, V., Millerioux, Y., Clastre, M., Simkin, A. J., Marais, E., CrĂšche, J., Giglioli-Guivarcâh, N. & Papon, N. (2011). Fluorescent protein fusions in Candida guilliermondii. Fungal Genet Biol 48, 1004â11. |
Â